Rare Disease Therapeutics Market to hit US$ 495.27 billion by 2033, (CAGR 13.8%)
Rare Disease Therapeutics Market Report 2024-2033 | CAGR, Key Players & Regional Insights
United States Rare Disease Therapeutics Market Analysis 2024-2033 | Biologics, Gene Therapy & Small Molecules”
AUSTIN, TX, UNITED STATES, December 5, 2025 /EINPresswire.com/ -- Market Size and Growth— DataM Intelligence 4Market Research LLP
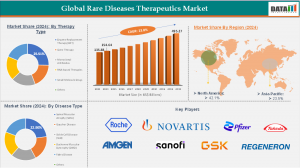
According to DataM Intelligence, the Global Rare Disease Therapeutics market was valued at USD 135.88 billion in 2023 and increased to USD 154.64 billion in 2024. It is projected to reach USD 495.27 billion by 2033, expanding at a compound annual growth rate (CAGR) of 13.8% between 2025 and 2033.
Growing diagnosis rates, advances in genetic medicine, strong pipeline of orphan drugs, and continued regulatory incentives such as FDA Orphan Drug Designation (ODD) are accelerating market expansion.
Precision medicine, gene therapies, RNA-based therapeutics, and monoclonal antibodies are reshaping treatment paradigms, enabling disease-modifying interventions for conditions historically considered untreatable.
Get a Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):– https://www.datamintelligence.com/download-sample/rare-disease-therapeutics-market
Growth Drivers
1. More than 350 million people worldwide are affected by rare diseases, with only 5% having FDA-approved treatments.
2. Over 1,050 rare disease drug candidates were in clinical pipelines in 2024, a 38% YoY rise.
3. Global orphan drug R&D spending surpassed USD 120 billion in 2024.
4. Gene therapy approvals doubled between 2023 and 2024, boosting investments in mRNA and cell-based therapeutics.
5. Reimbursement reforms in the U.S., EU, Japan, and China are accelerating patient access to costly therapies.
Market Segmentation Analysis
1. Biologics (Monoclonal Antibodies, Enzymes, Proteins): Held 52% market share (USD 124B) in 2024, projected to reach USD 318B by 2032, with strong presence in metabolic and neuromuscular disorders.
2. Gene & Cell Therapies: Accounted for 28% (USD 67B) in 2024, expected to grow fastest to USD 205B by 2032, supported by over 250 Phase II/III trials.
3. Small Molecules: Represented 18% (USD 43B) in 2024, forecasted to reach USD 87B by 2032, maintaining importance in oncology and hematology.
4. Others (RNAs, Peptides, Plasma-Derived): Made up 2% (USD 4.6B) in 2024, with growth to USD 14.4B by 2032, driven by increased use in immune and dermatological disorders.
By Disease Area
Rare Cancers – 34% share (USD 81 B in 2024)
Expected to exceed USD 220 B by 2032 driven by precision oncology.
Neurological Disorders – 26% share (USD 62 B)
Gene therapies driving treatment advances in SMA & Huntington’s.
Metabolic & Endocrine Disorders – 18% share (USD 43 B)
Demand driven by enzyme replacement therapies (ERT).
Blood Disorders – 12% share (USD 29 B)
Strong market for thalassemia & hemophilia.
Others (Dermatology, Immune, Pulmonary) – 10% share (USD 24 B)
By Route of Administration
Injectable / IV – 60%
Oral – 30%
Others (Subcutaneous, Inhaled) – 10%
Request for Customized Sample Report as per Your Business Requirement: https://www.datamintelligence.com/customize/rare-disease-therapeutics-market
Regional Insights
United States
The U.S. market was USD 104.5 billion in 2024 and will reach USD 273.6 billion by 2032 at 12.5% CAGR.
60% of global orphan drug approvals originate in the U.S.
Medicare and private payers expanded coverage for ultra-rare therapies.
300 cell & gene therapy trials active in 2025.
Japan
Japan’s rare disease therapeutics market was USD 21.4 billion in 2024 and will grow to USD 58.6 billion by 2032 at 13.3% CAGR.
Strong R&D support for lysosomal & metabolic disorders.
Fast-track review system reduced approval timelines by 35%.
Subsidized access for pediatric rare diseases.
Key Players
The market is highly competitive yet innovation-driven, with large pharma and biotech firms accelerating orphan drug pipelines.
Roche | Novartis | Pfizer | Takeda | Sanofi | Bristol Myers Squibb | Amgen | Vertex Pharmaceuticals | Ionis Pharmaceuticals | Sarepta Therapeutics | F. Hoffmann-La Roche Ltd, Novartis AG, Takeda Pharmaceutical Company Limited, Amgen Inc., Sanofi S.A. | GlaxoSmithKline plc | Regeneron Pharmaceuticals, Inc. | Biogen Inc.
Key Highlights
. Vertex crossed USD 10.4 billion rare disease revenue supported by cystic fibrosis and sickle cell therapy launches.
. Sarepta expanded gene therapy commercialization across the EU and APAC.
. Takeda and Pfizer jointly initiated global trials for rare hematology gene therapies.
. Roche acquired Prometheus Bioscience to strengthen immunology/rare pipeline.
Recent Developments
1. Vertex launched CRISPR-based therapy for sickle cell disease (February 2025)
2. Sarepta reported 65% improvement in ambulatory function in Duchenne muscular dystrophy Phase III trial (March 2025)
3. Pfizer inaugurated $500M gene therapy manufacturing center in North Carolina (January 2025)
4. Roche received FDA fast-track designation for rare pediatric epilepsy drug (December 2024)
Buy This Report with Year-End Offer (Buy 1 report: Get 30% OFF | Buy 2 reports: Get 50% OFF each! Limited time offer): https://www.datamintelligence.com/buy-now-page?report=rare-disease-therapeutics-market
Market Outlook & Opportunities
1. Biologics to remain dominant, exceeding USD 318 B by 2032
2. Gene and cell therapy to grow fastest—CAGR 16.9%
3. AI-enabled precision diagnostics will accelerate early detection
4. Partnerships between pharma and genetic sequencing companies will unlock new treatment pipelines
5. Asia-Pacific forecast to record highest CAGR (14.8%) due to regulatory modernization and clinical trial expansion
Conclusion
The Rare Disease Therapeutics Market is entering a breakthrough era shifting from symptomatic management to curative and disease-modifying treatments. With robust clinical pipelines, large-scale investments in gene therapy, and rapidly improving regulatory and reimbursement ecosystems, the industry will triple in value by 2032. Pharma giants and pioneering biotechs will continue to lead innovation toward improved patient survival, quality of life, and accessible care.
Related Reports
Rare Disease Genetic Testing Market
Rare Neurological Disease Drugs Market
Sai Kiran
DataM Intelligence 4market Research LLP
+1 877-441-4866
sai.k@datamintelligence.com
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.